Photodynamic therapy (PDT) free of side effects
Photodynamic therapy (PDT) Photogen
DongSeong Pharm Digital Healthcare
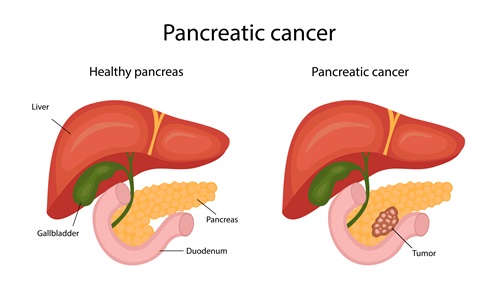
Pancreatic cancer remains a challenging cancer to cure. In fact, according to the 2020 national cancer statistics, the 5-year survival rate for pancreatic cancer is only an average of 15.2%.
The biggest challenge with pancreatic cancer is the lack of specific symptoms even when the disease is present. Various digestive organs surround the pancreas, so there are usually no significant symptoms until about 80% of the organ is damaged. Moreover, surgery is often complex due to its intricate connection with multiple blood vessels. Only about 20% of pancreatic cancer patients are eligible for surgery.
The limited treatment options available for pancreatic cancer patients pose another significant challenge.
In the realm of chemotherapy, for instance, after the approval of 'gemcitabine plus nab-paclitaxel' in 2013, it took a decade for Onivyde (irinotecan liposome injection) to be approved as a first-line therapy for metastatic pancreatic ductal adenocarcinoma.
However, Onivyde only reduced the risk of death by a mere 16%. Generally, if a chemotherapy regimen fails to reduce the risk of death by more than 20%, it is deemed ineffective.
■ Photodynamic therapy (PDT) is virtually free of side effects.
However, DongSeong Pharmaceuticals' photodynamic therapy (PDT) has received phase 2 clinical approval from the Korea Food and Drug Administration (KFDA), offering a ray of hope for pancreatic cancer patients.
Photodynamic therapy utilizes light as a treatment modality.
It requires three essential components: a light source, a laser, a photosensitizer, and oxygen.
When activated by an appropriate wavelength and intensity of light, the photosensitizer either directly acts on the tumor or generates reactive oxygen species to destroy it.
In PDT, the photosensitizer 'Photogen' is utilized.
Developed by DongSeong Pharmaceuticals, the domestic photosensitizer 'Photogen DSP-1944' boasts enhanced purity of active pharmaceutical ingredients (API) compared to imported drugs like Photolon, improving substance safety and stability.
The novel drug Photogen, along with PDT medical devices (laser irradiation equipment, laser diagnostic devices, etc.), is being researched worldwide for cancer treatment methods for PDT-PDD (photodynamic therapy-photodynamic diagnosis).
The most significant advantage of photodynamic therapy is its minimal side effects. Even if side effects occur, they are limited to skin photosensitivity due to the photosensitizer, which can be controlled.
■ Photogen, a domestically produced photosensitizer, achieves successful localization.
DongSeong Pharmaceuticals has been at the forefront of photodynamic therapy (PDT) research in South Korea for over a decade, evidenced by the establishment of the dedicated cancer research center, DongSeong Cancer Center, solely focused on PDT. The journey to obtaining approval for the recent Phase 2 clinical trial spanned 17 years, highlighting the company's steadfast commitment to advancing cancer treatment through innovative therapies.
According to DongSeong Pharmaceuticals, the Phase 2 clinical trial targets patients with unresectable locally advanced pancreatic cancer, evaluating the efficacy and safety of Photogen (DSP1944) injection as an additional treatment combined with chemotherapy.
Photogen, the photosensitizer utilized in the trial, has demonstrated utility in several previous studies.
In January of this year, DongSeong Pharmaceuticals presented research findings on Photogen at the 17th Korean Peritoneal Cancer Society Congress, showcasing significant advancements in photodynamic diagnosis (PDD) using Photogen activation at 405nm. The study revealed a substantial improvement in the diagnostic accuracy of laparoscopic examination compared to conventional white light examination, as demonstrated by enhanced sensitivity and specificity.
Furthermore, Photogen's research achievements have been recognized in prestigious scientific journals such as the International Journal of Molecular Sciences and Scientific Reports, underscoring its impact on the scientific community. A representative from DongSeong Pharmaceuticals expressed their dedication to accelerating research efforts since the establishment of the Daegu Cancer Center in 2017, emphasizing plans further to explore the application of Photogen in pancreatic cancer PDT
and peritoneal cancer PDD studies in the future.
[Mini Interview] Lee Yang-Goo, CEO of Dong Sung Pharmaceutical

- You obtained Phase 2 Clinical Trial Approval from the Ministry of Food and Drug Safety.
For over 15 years, DongSeong Pharmaceuticals has been the sole pioneer in photodynamic therapy (PDT) cancer treatment research and development in South Korea.
Consequently, this recent clinical approval marks a significant milestone. PDT involves administering photosensitizers into the bloodstream, which accumulate in cancerous tissues, followed by selective destruction of cancer cells using laser light of specific wavelengths.
It has minimal impact on normal tissues and allows for rapid recovery when administered laparoscopically.
- What are the objectives of Phase 2 Clinical Trial?
The clinical trial is expected to span one year. It will involve 40 patients with metastatic pancreatic cancer conducted at Severance Hospital. DongSeong Pharmaceuticals plans to gradually expand the indications from pancreatic cancer to include breast cancer, cervical cancer, and peritoneal cancer, among
others, making it the first in the world to explore the effectiveness across
various cancer types.
- Given the dedication to PDT over the past 17 years, numerous challenges might exist.
Securing approval for the PDT photosensitizer drug posed significant hurdles. Until now, photosensitizers have been imported through Belarus. However, obtaining approval from the Korean Food and Drug Administration (KFDA) was necessary for domestic use. To achieve this, extensive efforts were made, including on-site inspections of the BMP factory and optimization of production processes. As a result, we successfully developed our photosensitizer, "Ponogen," tailored for the Korean market. Clinical trials were conducted to establish its efficacy and safety, ultimately leading to regulatory approval.
- Do you have any words for pancreatic cancer patients?
As cancer cases continue to rise worldwide, with over 250,000 diagnoses annually in South Korea alone and pancreatic cancer affecting around 7,500 patients each year, it is crucial to acknowledge the severity of the situation. Pancreatic cancer, in particular, presents a dismal overall survival rate. With this clinical trial, we aim to offer hope to pancreatic cancer patients who face significant unmet medical needs. We are committed to doing our utmost to provide a beacon of hope for those battling this challenging disease.